
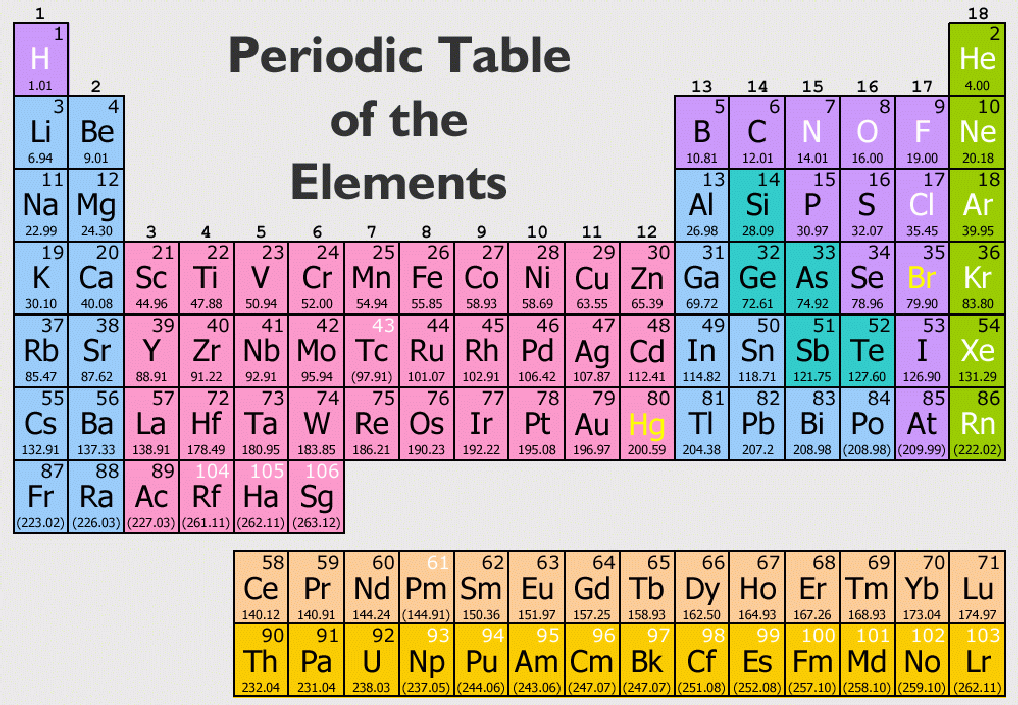
The periodic table on the left separates elements into three groups: the metals (green in the table), nonmetals (orange), and metalloids (blue). The 17 nonmetal elements are: hydrogen, helium, carbon, nitrogen, oxygen, fluorine, neon, phosphorus, sulfur, chlorine, argon, selenium, bromine, krypton, iodine, xenon, and radon. Nonmetals are located on the far right side of the periodic table, except hydrogen, which is located in the top left corner. Keeping this in view, where are the nonmetals on the periodic table? Some elements appear in both metallic and non- metallic forms. The number that tells the number of valence electron is the GROUP NUMBER.Īdditionally, how many metals and nonmetals are in the periodic table? About 91 of the 118 elements in the periodic table are metals the others are nonmetals or metalloids. The metals are found at the left side of the periodic table, the non metals are found at the middle part of the periodic table and the noble gases are found at the right side. Subsequently, one may also ask, where can you find metals nonmetals and noble gases on the periodic table? My results and conclusion don't match up entirely, but my other findings line up to the information on the chart.Metals are located on the left of the periodic table, and nonmetals are located on the upper right. Do your results and conclusions agree with the information found on this chart? I agree with this chart.
The four metals we used, in order from most reactive to least reactive, are Zinc, Magnesium, Calcium, and Tin.

Use data from your lab to support your answer. List the four metals from most reactive to least reactive.The Magnesium was in a strip of solid metal, while the calcium was in a powdery form. sequential) These are the transition metals f-block is located below the main table. What might be a reason for the difference in behavior between magnesium and calcium when placed in water? Magnesium and Calcium probably reacted differently when placed in the water because of the forms that they were in. The Periodic Table: Our current periodic table lists 109 elements.Add enough HCl to completely cover each sample (use a disposable pipette). Obtain a dropper bottle of HCl from Charlene. Obtain your samples from Charlene, and place them in your labelled test tubes. Record your data and observations in the table below. Add enough water to cover the sample (use a disposable pipette). Get your samples from Charlene, and put them in your labelled test tubes. What element is in Group 3 and in the 3rd period? The element in Group 3 and Period 3 is Aluminum.What are the names of two metal families? In Group 1 are the Alkali Metals and in Group 2 are the Alkaline Earth Metals.In general, where are metals located on the Periodic Table? The metals are located to the left of the Metalloid Boundary.Families are specific groups on the Periodic Table. The horizontal rows on the Periodic Table are periods. What are groups? What are periods? What are families? The vertical columns on the Periodic Table are groups.To explore the reactivity trends of metals in groups and periods of the Periodic Table.


 0 kommentar(er)
0 kommentar(er)
